
The eighth-annual Emerald Conference, an interdisciplinary cannabis science event, was an intellectual celebration of the cannabis plant for the inquisitive, data-hungry minds of the industry. Hosted over three days on the tranquil shores of Coronado in San Diego, this year’s Emerald Conference played host to hundreds of attendees, more than fifteen speakers, and approximately fifty exhibitors and sponsors with booths and technical poster presentations. Overall, the industry event succeeded in sparking meaningful conversations about the significant role data and science are playing in the evolution of the industry.
“The Emerald Conference is important because it provides a platform for scientific communication and collaboration,” said David Dawson, Ph.D., chief scientific officer at Via Innovation. “Open scientific dialogue is important for all fields, but especially an emerging field such as cannabis science. To overcome the black market paranoia and misinformation that has run rampant in this field until recent decades, scientists need to come together and share their findings with their peers in the scientific community.”
For first-time attendee Corrie N. Purser, who holds a bachelor’s degree in chemistry and a master’s in biochemistry, the conference represented the perfect opportunity to gather with like-minded leading scientists in the analytical testing field while representing her employer, GERSTEL, a provider of sample extraction solutions and automated sample preparation for gas chromatography/mass spectrometry (GC-MS) and liquid chromatography/mass spectrometry.
“Incorporating olfactory detection unlocks a whole new world of sensory analysis of cannabis products, allowing the sensing of compounds by the human nose as they elute from the column of a GC-MS,” Purser said. “It will be exciting to see how a versatility of analytical testing techniques will change the landscape of the science being performed within this industry. I can see automation, thermal desorption, and olfactory detection all playing a major role in addressing challenges in the analytical field of cannabis testing.”
While the future of the industry was at the heart of the event’s discussions, some sessions were able to highlight the excellent work being accomplished despite the restrictions and limited resources available to today’s researchers.
We’ve already made great strides
After Biden signed the Medical Marijuana and Cannabidiol Research Expansion Act into law, the industry feels poised to make meaningful advances in cannabis research that could improve the lives of millions of patients across the country and open new opportunities for plant-touching businesses willing to participate in research and development.
While many of the speakers highlighted the need for federal assistance to deschedule cannabis and remove other roadblocks to meaningful research, the Friday morning presentation by Bonni Goldstein, M.D., on cannabis use in autism spectrum disorders demonstrated the immense success many patients already are experiencing with cannabis as medicine, along with the data to back it up. Goldstein has been a licensed physician for more than thirty years. She’s a published author and active researcher who has treated more than 2,000 pediatric patients since shifting her professional focus to cannabis medicine about fifteen years ago.
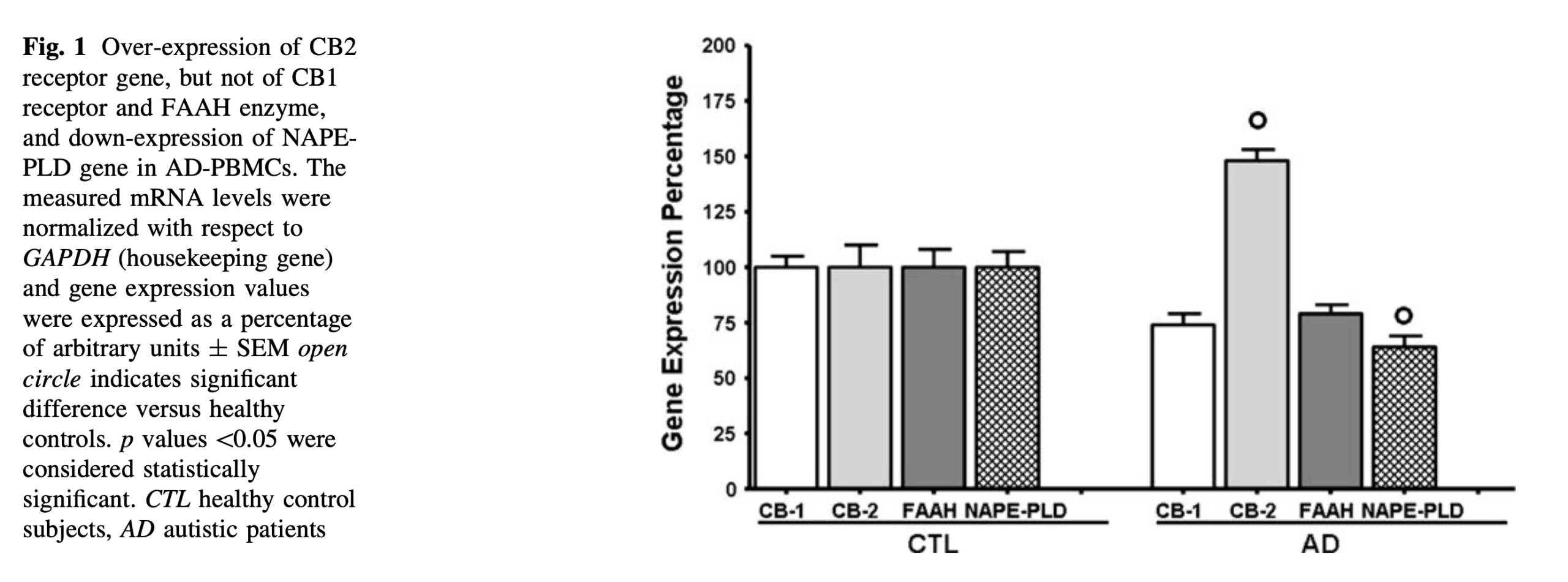
According to Goldstein, autism affects one in forty-four children. Those children currently have access to only two FDA-approved “antipsychotic drugs” designed to treat irritability without addressing the root cause, which she and other researchers believe to be an imbalance of the endocannabinoid system. Objective evidence of an imbalance can be seen in multiple studies, one of which shows non-autism children exhibit 100-percent gene expression of different components of the endocannabinoid system, while the autistic group shows an imbalance of the same components like CB-1 and CB-2 receptor genes that can be modulated with cannabis in an effort to achieve homeostasis.
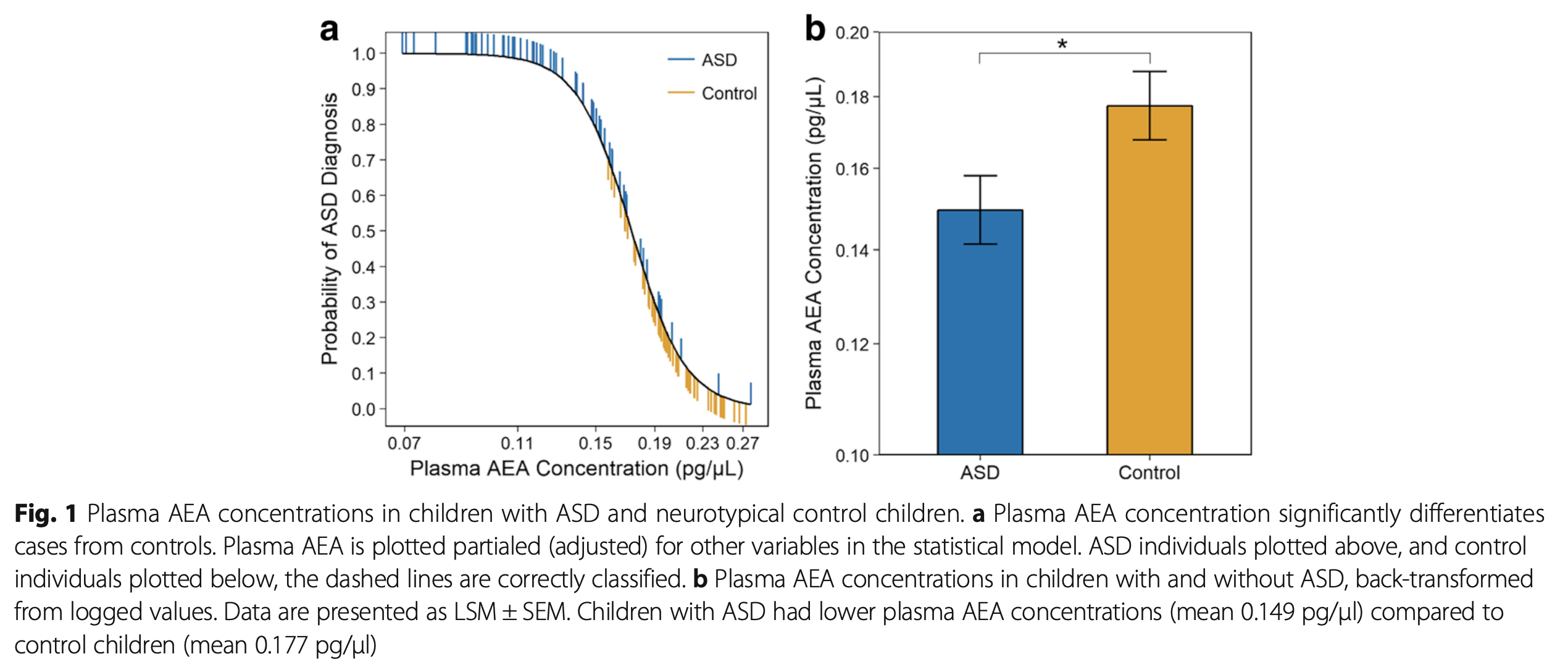
A more recent Stanford University study found plasma anandamide concentrates are lower in children with autism spectrum disorder (ASD), indicating children with lower anandamide concentrations were more likely to experience ASD, according to the researchers.
“These findings are the first empirical human data to translate preclinical rodent findings to confirm a link between plasma anandamide concentrations in children with ASD,” the researchers wrote. “Although preliminary, these data suggest that impaired anandamide signaling may be involved in the pathophysiology of ASD.”
While measuring plasma anandamide concentrates is difficult, the results could be used as a biomarker for early diagnosis. According to Goldstein, when researchers in the study were presented with the anandamide levels of the control and test groups without any other information, they were able to identify which subjects had been diagnosed with ASD.
While measuring plasma anandamide concentrates in the blood may be difficult, if not completely impractical, other options are emerging to accomplish the same goals, with promising results.
The future is bright
Goldstein recently conducted a small preliminary study in the field of pharmacometabolomics with Cannformatics co-founders Itzhak Kurek, Ph.D., and Ken Epstein. The research team, which has published two papers in the past six months, looked at the impact of medical cannabis on children who use it to alleviate the symptoms of autism under medical and parental supervision. Specifically, they studied the cannabis-responsive biomarkers that are easily collected in saliva to measure pathways involved in neurological dysfunction, pain, aggression, and inflammation.
While human DNA can provide a picture of the potential for development, it cannot tell researchers what’s happening at any given moment or how an individual is affected by a medication. However, the biomarkers under examination for Goldstein’s study can provide researchers with objective data that shows what’s actually happening in the subject’s body, “allowing clinicians to predict the efficacy and toxicity of a drug treatment and also personalize treatment for a specific individual,” according to Goldstein’s presentation. With sampling performed by saliva, researchers quickly can identify a patient’s baseline, where they are after treatment, and what happens if they stop a treatment.
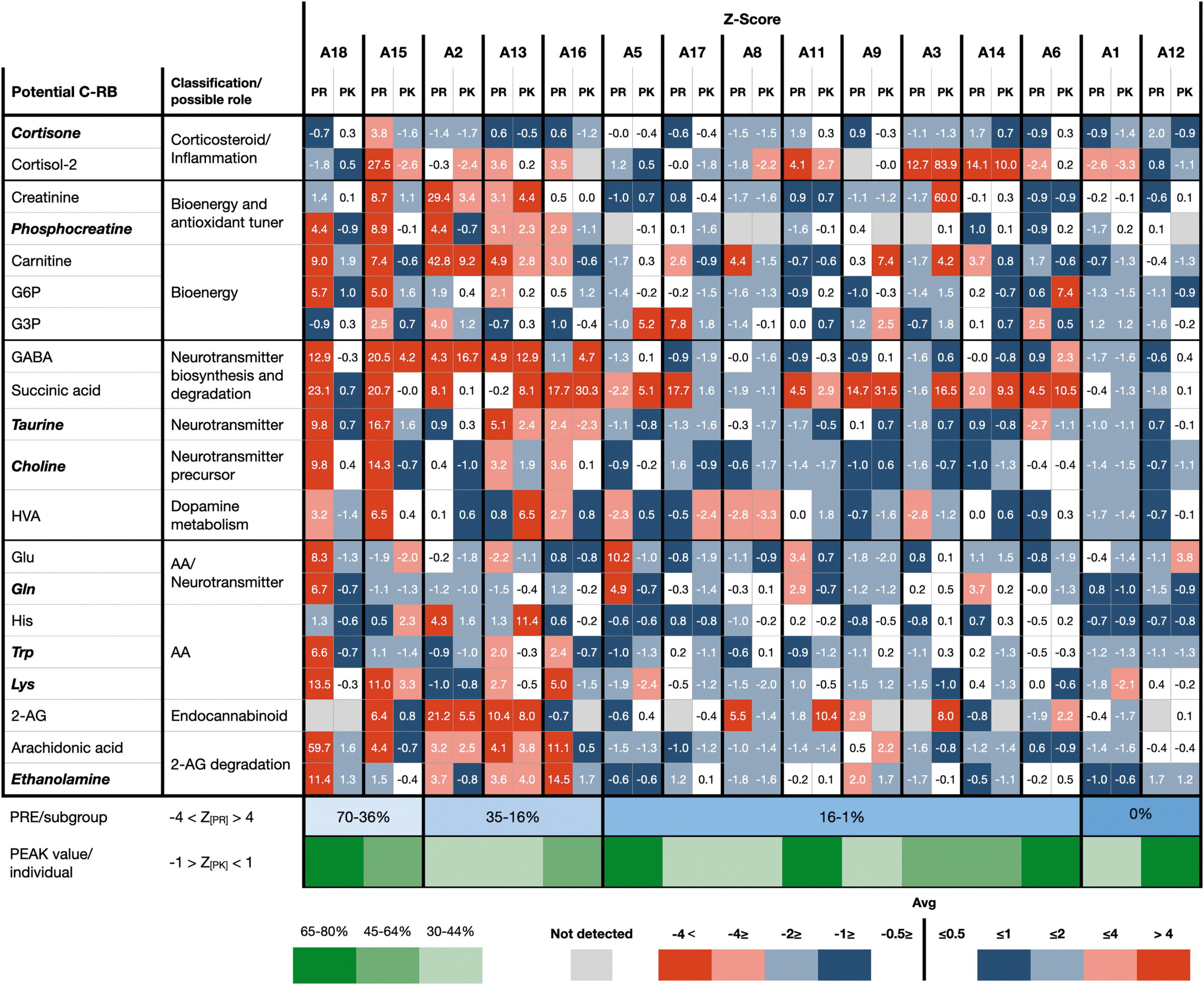
The small sample size of eighteen children serves as a compelling proof of concept for the larger, 150-patient group study being planned for 2023. Although the preliminary study was not able to associate a patient’s cannabis regimen with specific biomarker responses, Goldstein indicated there was some signaling.
The opportunity in research-grade cannabis
Thursday’s key panel on the current state of cannabis research in the U.S. and the implications of new reform covered a critical element missing from industry researchers’ box of tools: reliable sources for research-grade cannabis that are representative of the modern marketplace. According to the panel of Wes Burk, Jeff Keller, AC Braddock, and Mathew Indest, we still need to address the lack of standards and consistency in cannabis products to conduct meaningful, peer-reviewed research.
From 1968 to 2021, the University of Mississippi was the sole licensed supplier of federally approved cannabis for research. For those who’ve seen what’s been produced on the Mississippi farm, the results can be fairly described as underwhelming.
A report from PBS indicated moldy samples and materials that did not look or smell like cannabis according to scientists working on an FDA-approved trial in 2017. What’s currently available through the National Institute on Drug Abuse (NIDA) and defined as research-grade marijuana, presents THC levels between 2 and 7 percent with no mention of terpenes and only four minor cannabinoids. The NIDA is selling these materials for nearly $11 per joint and $2,500 per kilogram, which comes out to roughly $1,136 per pound—not a terrible wholesale price for state-licensed producers in today’s economy.
Unlike pharmaceutical companies investing heavily in research to develop countless failed drugs with the occasional big hit, many of the cannabis industry’s best cultivators are perfecting unique cannabinoid- and terpene-rich cultivars that can’t miss in the marketplace and likely check all the boxes for “research-grade cannabis” in terms of quality, variety, potency, consistency, and availability far better than what’s currently offered by the NIDA. One can only imagine the financial potential of producing a cultivar for recreational consumers that clinical trials later prove effectively treats PTSD, autism, or any number of medical conditions affecting millions of individuals. Look no further than the early days of Charlotte’s Web to understand what merely scratching the medical treatment surface can deliver.
For many indoor brands that place an emphasis on cultivating clean, consistent products with repeatable effects season after season, it may be worth considering research scientists as a new customer base with some highly aspirational goals. While the limited sales for clinical trials will not impact a brand’s financial position, they also won’t come at a substantial loss based on the NIDA’s pricing. Holding the cultivation keys to an effective treatment for a major medical condition is certain to change a brand’s trajectory, if not the entire industry, for many years to come.
